About WHI
The Women’s Health Initiative (WHI) is a long-term national health study funded by the National Heart, Lung, and Blood Institute, or NHLBI. The original WHI study began in the early 1990s and concluded in 2005. Since 2005, the WHI has continued as Extension Studies, which are annual collections of health updates and outcomes in active participants. The second Extension Study enrolled 93,500 women in 2010 and follow-up of these women continues annually.
As with the original WHI study, the main areas of research are cardiovascular disease, cancers, and osteoporotic fractures. While WHI continues to focus on strategies to prevent the major causes of death, disability, and frailty in older women, the breadth and richness of the WHI data allow for the exploration and investigation of many more research questions on women’s health and aging.
To learn more about the original WHI study that began in the early 1990’s, including specific details about the three clinical trials and the observational study, visit the WHI program page on the NHLBI website.
About WHI Extension Participants
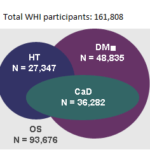
then (n= 161,808) and now
(n= 47,503)
(n=161,808)
Not Hispanic/Latina, n=153,117 (94.6%)
Hispanic/Latina, n=7,312 (4.5%)
Unknown/Not reported, n=1,379 (0.9%)
(n=47,503)
Not Hispanic/Latina, n=45,500 (95.8%)
Hispanic/Latina, n=1,883 (4.0%)
Unknown/Not reported, n=120 (0.3%)
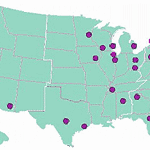
Active Participants by State
| State | Participants |
|---|---|
| California | 7,071 |
| Florida | 2,998 |
| Massachusetts | 2,904 |
| New York | 2,532 |
| North Carolina | 2,319 |
| Wisconsin | 2,111 |
| Ohio | 1,818 |
| Texas | 1,804 |
| Illinois | 1,530 |
| Minnesota | 1,332 |
| Arizona | 1,260 |
| Maryland | 1,214 |
| Georgia | 1,198 |
| Washington | 1,175 |
| NewJersey | 1,167 |
| Iowa | 1,103 |
| Oregon | 1,072 |
| Pennsylvania | 989 |
| Michigan | 949 |
| Hawaii | 842 |
| Alabama | 800 |
| Rhode Island | 796 |
| Nevada | 795 |
| Tennessee | 662 |
| Virginia | 643 |
| District of Columbia | 427 |
| South Carolina | 262 |
| Connecticut | 244 |
| Colorado | 222 |
| New Hampshire | 202 |
| Kentucky | 169 |
| Mississippi | 144 |
| Maine | 142 |
| Indiana | 125 |
| Idaho | 92 |
| Utah | 87 |
| Missouri | 86 |
| Arkansas | 85 |
| New Mexico | 73 |
| Delaware | 66 |
| Vermont | 65 |
| Montana | 41 |
| West Virginia | 37 |
| Kansas | 34 |
| Oklahoma | 30 |
| Nebraska | 26 |
| Louisiana | 23 |
| Wyoming | 16 |
| South Dakota | 15 |
| Alaska | 12 |
*States with fewer then 10 ppts not included
Journey through WHI’s history
WHI Leadership – Steering Committee

Steering Committee Chair
Fred Hutchinson Cancer Center

Fred Hutchinson Cancer Center

Fred Hutchinson Cancer Center

National Heart, Lung, and Blood Institute

National Heart, Lung, and Blood Institute

University of Massachusetts

Stanford University

Wake Forest University

University at Buffalo

University of Alabama at Birmingham

Albert Einstein College of Medicine

The Ohio State University

University at Buffalo

University of North Carolina
An organizational chart provides further information detailing the roles of individual WHI Steering Committee members. Please continue reading the next section to learn more about former PIs and key contributors to the Women’s Health Initiative over the past three decades.
Study Organization
The WHI study consisted of 40 Clinical Centers, several with additional satellite locations, during the recruitment and active intervention phase of WHI (1993-2005). During the WHI Extension Studies, the Clinical Centers, shown in the interactive map below, were consolidated into 10 Regional Centers in 2010. The WHI Clinical Coordinating Center (CCC) is located at the Fred Hutchinson Cancer Research Center, in Seattle, WA. The tables below show the investigator names and locations of the original Clinical Centers and the current Regional Centers. Visit the Contacts page for CCC and Regional Center contact information.
Current Study Organization
WHI Project Office
| Institution | Location | Project Officer |
|---|---|---|
| National Heart, Lung, and Blood Institute | Bethesda, MD | Jacques Rossouw, M.D., 1993-2014 Shari Ludlam, M.P.H., 2014-2020 Jared Reis, Ph.D., 2020- |
WHI Clinical Coordinating Center
| Institution | Location | Principal Investigator |
|---|---|---|
| Fred Hutchinson Cancer Research Center | Seattle, WA | Ross Prentice, Ph.D. 1993-2010 Garnet Anderson, Ph.D. 2010- |
WHI Regional Centers
| Region | Regional Center or Regional Center Site | Institution | Principal Investigator |
|---|---|---|---|
| Northeast | Buffalo, NY Boston, MA | University at Buffalo Brigham and Women’s Hospital | Jean Wactawski-Wende, Ph.D. JoAnn Manson, M.D., Dr.P.H. |
| Southeast | Winston-Salem, NC | Wake Forest University | Mara Vitolins, Dr.P.H. |
| Midwest | Columbus, OH | The Ohio State University | Electra Paskett, Ph.D. |
| West | Stanford, CA | Stanford University | Marcia Stefanick, Ph.D. |
| CCC | Seattle, WA | Fred Hutchinson Cancer Research Center | Garnet Anderson, Ph.D. |
Legacy Investigators
These WHI Investigators have a history of major contribution to WHI 5 or more years ago as Past Principal Investigator or Co-Investigator at the CCC, a Regional Center, or an original WHI site or while employed at the NIH. Past WHI chairs of a WHI standing committee also may qualify as Legacy Investigators.
| Name | Institution |
|---|---|
| Shirley Beresford | University of Washington |
| Margery Gass | Cleveland Clinic |
| Dorothy Lane | Stony Brook University |
| Erin LeBlanc | Center for Health Research, Kaiser Permanente Northwest |
| Karen Margolis | Health Partners |
| Mary Jo O’Sullivan | Jackson Memorial Hospital |
| John Robbins | University of California – Davis |
| Jennifer Robinson | University of Iowa |
| Haleh Sangi-Haghpeykar | Baylor College of Medicine |